In Alzheimer’s disease, the nerve cells cease functioning as they should, which leads to a deterioration of memory and learning. AlzeCure has identified drug-like compounds that stimulate neurotrophic signaling pathways, thereby strengthening nerve cell function and improving memory.
NeuroRestore is a platform of symptomatic drug candidates for diseases where cognitive ability is impaired, such as Alzheimer’s.
NeuroRestore stimulates several important signal pathways in the brain, which among other things leads to improved cognition. In preclinical studies with NeuroRestore, we have been able to demonstrate that our drug compounds not only boost communication between nerve cells but also improve cognitive ability.
The drug candidates in NeuroRestore stimulate signaling of neurotrophins, the most well-known of which is Nerve Growth Factor (NGF) and Brain Derived Neurotrophic Factor (BDNF). These neurotrophins are important for maintaining nerve cell function and communication, which are impaired with cognitive disorders. BDNF plays an important role for nerve cell function and communication in the areas of the brain that are essential for our cognitive ability, such as the hippocampus, located in the temporal lobe.
The reduced function impairs both the communication between the contact surfaces of the nerve endings and the function of the nerve cells, which gives rise to the cognitive impairments. There is also genetic support for this target mechanism – a genetic variation of BDNF in humans, leading to a reduced secretion of BDNF, is involved in cognitive decline related to both neurodegenerative processes seen in Alzheimer’s and Parkinson’s disease, but also in other cognitive indications such as traumatic brain injury and sleep disorders. AlzeCure assesses that there is also potential to be able to add additional indications based on the specific target mechanism. In the preclinical trials, ACD856, the lead drug candidate in the NeuroRestore platform, has been able to demonstrate that it can enhance signaling in the intended signaling pathway and improve cognitive ability. Among other things, the substance has been able to show that it can reverse age-induced memory impairments and enhance the effect of existing drugs (acetylcholinesterase inhibitors), something that AlzeCure sees as a competitive advantage.
Based on the specific target mechanism for NeuroRestore, there is also a potential for disease-modifying effects with these substances, i.e. that the underlying disease process is affected by the treatment. This as both the neurotrophins NGF and BDNF play important roles in maintaining normal function and development of nerve cells, but also to protect them from damage, so-called neuroprotective effects. Nerve cell death is clearly correlated to functional impairment in Alzheimer’s patients and there are currently no marketed drugs with these protective effects.
This area has received an increased focus in the company and new preclinical data within the NeuroRestore platform shows, among other things, positive effects on mitochondrial function, something that is disturbed in neurodegenerative diseases such as Alzheimer’s. These preclinical studies also seem to indicate that substance treatment leads to increased survival for the nerve cells. During 2022 and 2023, the studies have been supplemented with additional data on the neuroprotective, regenerative and long-term effects of ACD856. Furthermore, the data show that ACD856 increases the amount of a specific protein that plays an important role in nerve cell communication, something that is strongly affected in the disease. These important data, which further strengthen NeuroRestore’s potential as a disease-modifying treatment, have been presented at several scientific conferences during the last two years.
There is also strong scientific support for this target mechanism in depression. NeuroRestore substances have had effects in preclinical models of depression (1) which are further supported by data in recent articles in the reputable journals Cell (2), Nature (3) and Science (4). These studies show that several different classes of antidepressants appear to mediate their effects via BDNF/TrkB, further strengthening the link between BDNF and depression. AlzeCure has been able to show in preclinical models that NeuroRestore substances have antidepressant effects and that they also release signaling substances in the brain that are relevant for depression.
NeuoRestore compounds have demonstrated efficacy in preclinical models for depression, which has been further supported by data in a recently published article in the highly respected publication Cell1.In the preclinical trials, ACD856, the leading drug candidate in the NeuroRestore platform, has been able to demonstrate that it can strengthen signaling in the intended pathway and improve cognitive ability. Among other things, the compound has been able to show that it can reverse age-induced memory impairment and strengthen the effect of existing drugs (acetylcholinesterase inhibitors), which AlzeCure views as a competitive advantage.
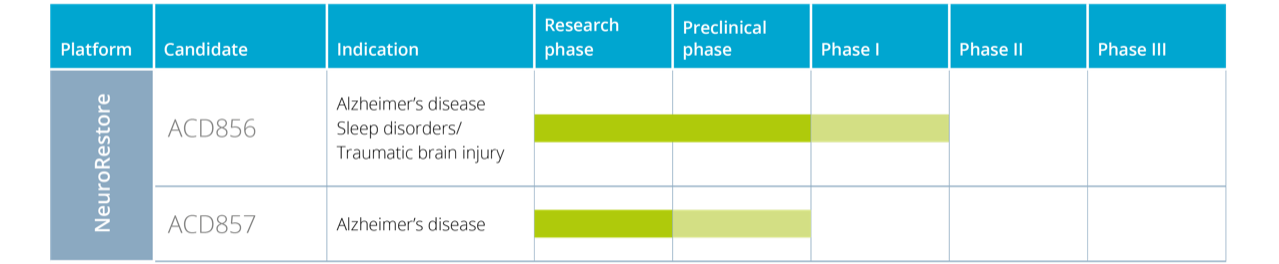
Within NeuroRestore, a new generation of symptom-relieving drugs is being developed for the treatment of cognitive dysfunction (memory disorders) in Alzheimer’s. The company initiated the first clinical study with the primary drug candidate in NeuroRestore, ACD856, at the end of 2019. The study was completed during the second quarter of 2020 according to plan. The results showed that ACD856 was well suited for further clinical development and thus further clinical studies could be initiated at the end of 2020, the so-called SAD study, also according to plan. The MAD study was also initiated in the third quarter of 2021, and these two studies, which are part of the Phase 1 program for the drug candidate, have as their primary purpose to evaluate its safety and tolerability in humans.
The MAD study, which was completed according to plan in June 2022, showed that ACD856 has a good tolerability and safety profile in humans. Furthermore, the results demonstrated that the substance had suitable pharmacokinetic properties with rapid uptake into the body, but also that ACD856 crosses the blood-brain barrier well and can be measured in the spinal fluid, which is important data that supports the further clinical development work. Data from so-called quantitative EEG also showed that the substance not only crossed the blood-brain barrier but also activated relevant nerve pathways in the brain. ACD857 is in the research phase and also has cognitive dysfunction / Alzheimer’s disease as the primary indication.
Find all our NeuroRestore publications HERE
Below you find some of the latest publications:
1. Madjid N, Lidell V, Nordvall G, Lindskog M, Ögren SO, Forsell P, Sandin J. Antidepressant effects of novel positive allosteric modulators of Trk-receptor mediated signaling – a potential therapeutic concept? Psychopharmacol (Berl). 2023 Aug;240(8):1789-1804.
2. Casarotto PC, Girych M, Fred SM, Kovaleva V, Moliner R, Enkavi G, Biojone C, Cannarozzo C, Sahu MP, Kaurinkoski K, Brunello CA, Steinzeig A, Winkel F, Patil S, Vestring S, Serchov T, Diniz CRAF, Laukkanen L, Cardon I, Antila H, Rog T, Piepponen TP, Bramham CR, Normann C, Lauri SE, Saarma M, Vattulainen I, Castrén E. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell. 2021 Mar 4;184(5):1299-1313.
3. Moliner R, Girych M, Brunello CA, Kovaleva V, Biojone C, Enkavi G, Antenucci L, Kot EF, Goncharuk SA, Kaurinkoski K, Kuutti M, Fred SM, Elsilä LV, Sakson S, Cannarozzo C, Diniz CRAF, Seiffert N, Rubiolo A, Haapaniemi H, Meshi E, Nagaeva E, Öhman T, Róg T, Kankuri E, Vilar M, Varjosalo M, Korpi ER, Permi P, Mineev KS, Saarma M, Vattulainen I, Casarotto PC, Castrén E. Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat Neurosci. 2023 Jun;26(6):1032-1041.
4. https://www.science.org/content/article/psychedelic-inspired-drugs-could-relieve-depression-without-causing-hallucinations
For more information and material: see here
The video below briefly shows how a compound from the NeuroRestore program acts in the brain.
ACD856 hypothesized mechanism of action
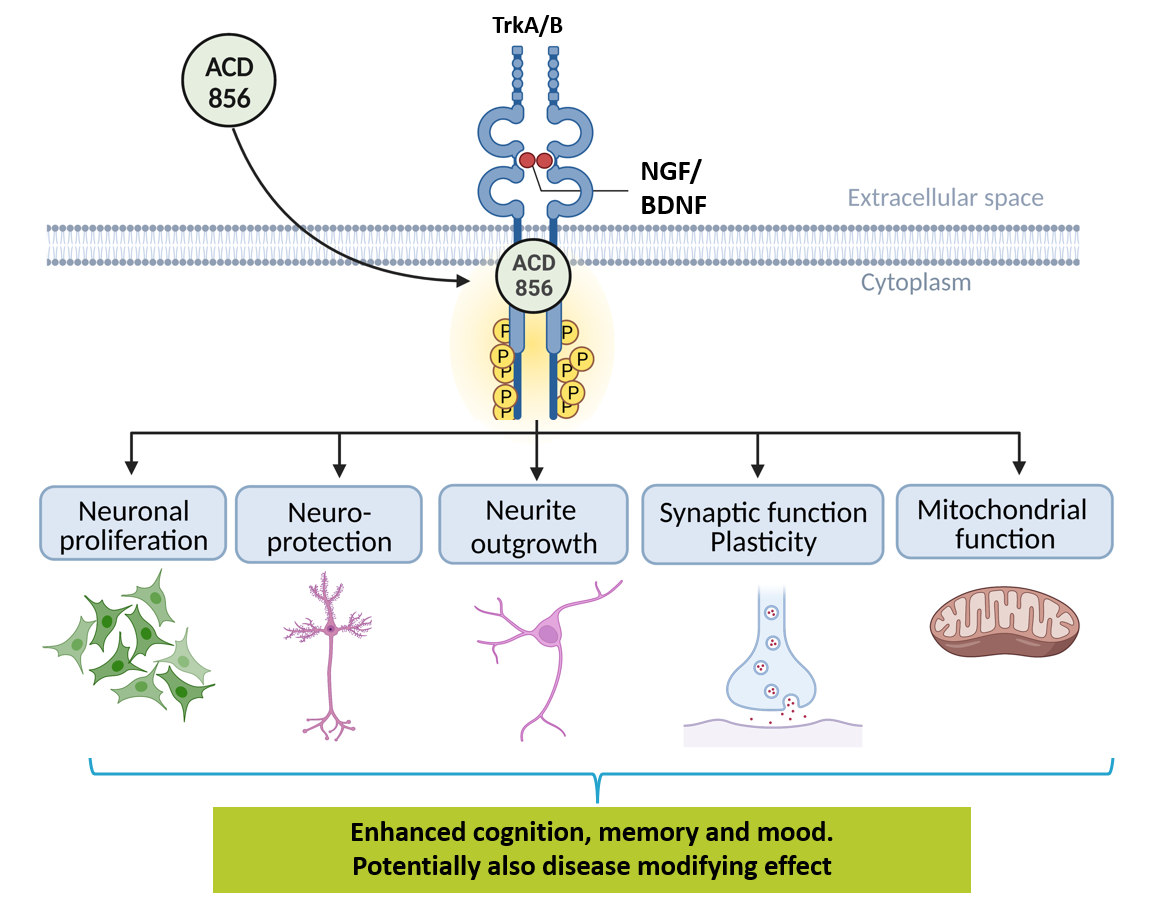
ACD856 is a novel small molecule positive modulator of Trk-receptors enhancing the signaling of neurotrophins such as NGF and BDNF. The enhanced signaling leads to short term symptomatic effects with long term benefits.
The long-term effects of ACD856 could include improved neuronal function, increased mitochondrial function, enhanced synaptic plasticity as well as improved cognition.
This would suggest a potential for both symptomatic and disease modifying effects for this class of compounds.