The TrkA-NAM project, which is in the pre-clinical research phase, is aimed at treatment of pain and has strong preclinical and clinical validation.
For the TrkA-NAM drug project, we have leveraged our knowledge concerning the underlying biology for the NeuroRestore platform in order to develop new compounds that focus on providing pain relief in conditions associated with severe pain. The goal of the project is to develop a small molecule “TrkA-negative allosteric modulator” that can reduce movement-induced and spontaneous pain in patients with painful osteoarthritis. The global osteoarthritis market is expected to reach USD 11.0 billion by 2025, from USD 7.3 billion in 2020. Growth in this market is driven by factors such as the increasing occurrence of osteoarthritis, the growing aging population, and an increase in the number of sports injuries.
Approximately 400 million people worldwide suffer from painful and activity-limiting osteoarthritis of the hip or knee. Many patients experience insufficient pain relief or side effects with current treatment, which today usually consist of NSAIDs or opiates, and there is a great need for more effective and better tolerated drugs in this field. In addition, there is a risk of abuse and development of tolerance even with short-term use of opioids.
Over the past decade, a number of anti-NGF antibodies have been developed and used in several clinical trials to treat painful osteoarthritis. The first positive study was with Tanezumab, which showed a potent analgesic effect in osteoarthritis of the knee in a phase II clinical trial, which has been followed by several phase III clinical trials for various pain indications. However, a small number of patients who received anti-NGF antibodies developed side effects, which has put the brakes on further development of these drugs.
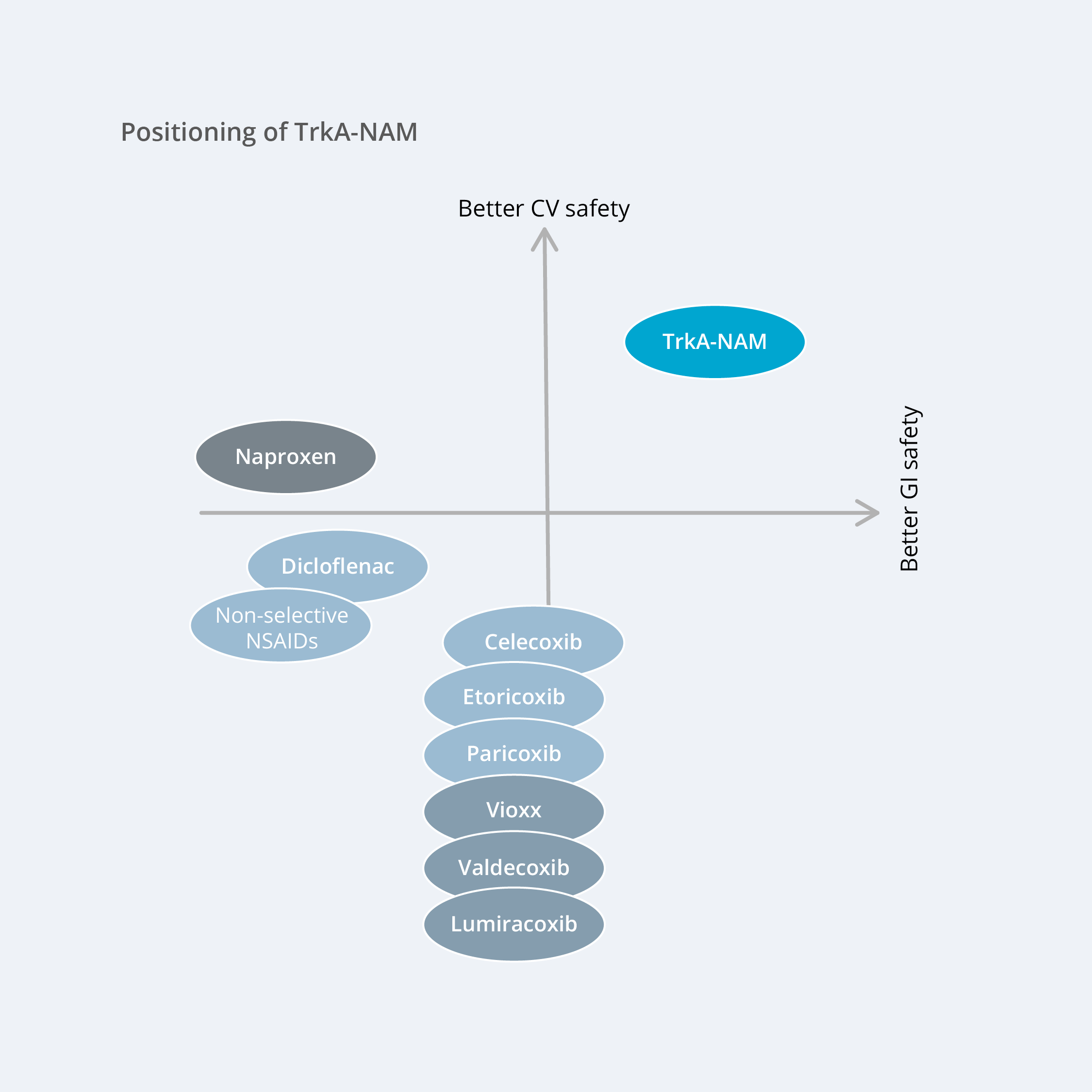
A small-molecule drug with a mechanism that generates the same favorable effects as anti-NGF-antibodies, but without the side effects observed for them, would have great market potential.
A selective TrkA-negative allosteric modulator meets these criteria. As previously mentioned, the target mechanism has been strongly validated by both preclinical and clinical data, and AlzeCure’s unique compounds differentiate themselves with their selective effect on relevant signaling pathways to achieve optimal pain relief without inducing side effects. In addition, the TrkA-NAM compounds are small molecules, which facilitates administration for patients (tablets) while contributing to more cost-effective treatment. Moreover, the product is non-opioid, an important consideration with respect to gaining future regulatory approval from authorities, including the FDA.
In January 2024, AlzeCure selected a drug candidate for the project, ACD137, which has now begun the next development phase, which includes pre-clinical safety studies – a requirement before clinical studies can begin.
The team at AlzeCure has many years of research experience in the fields of neurology and pain. This project is an excellent example of leveraging synergies between the projects and maximizing shareholder value.