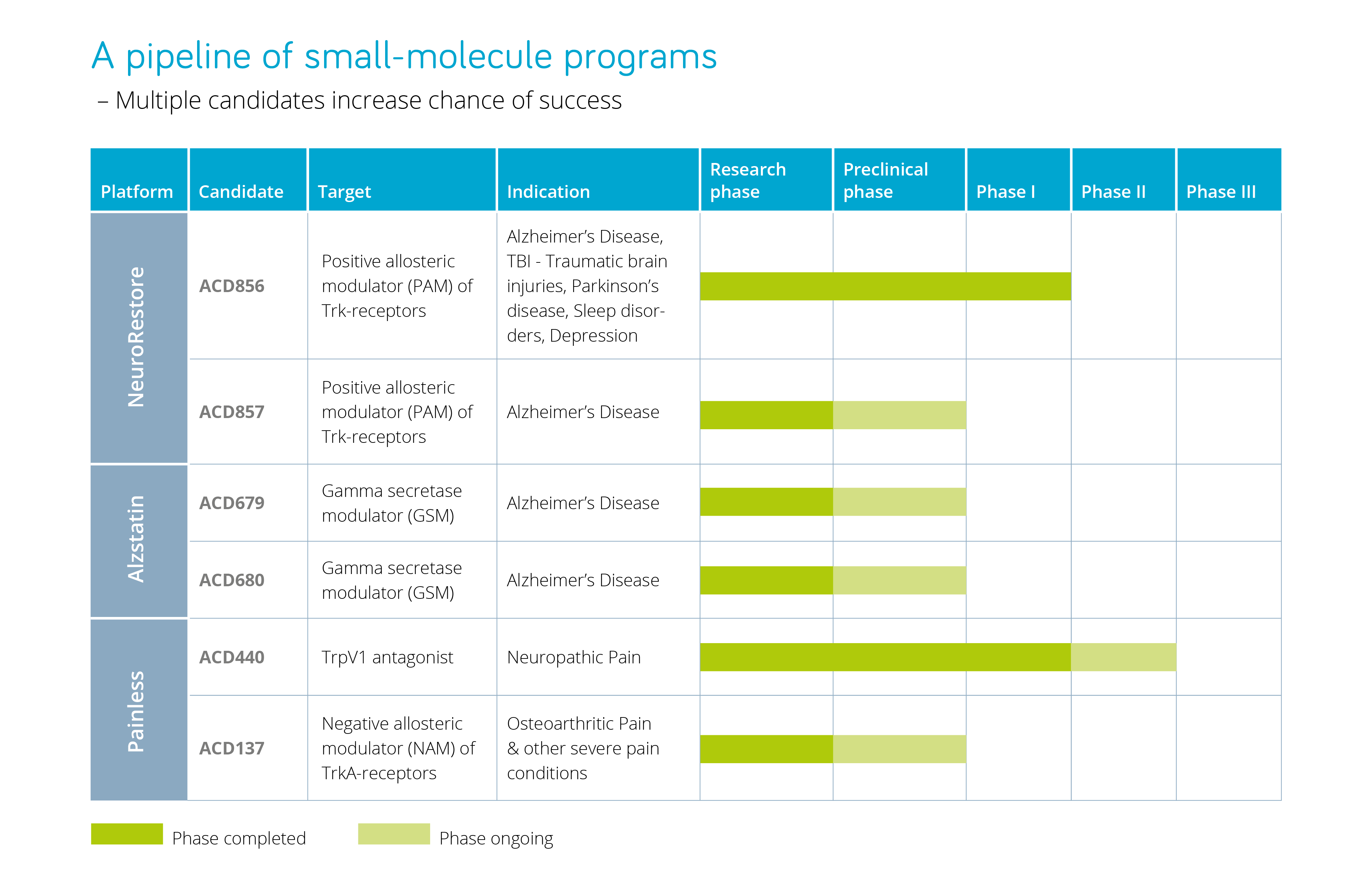
AlzeCure® works with several research platforms: NeuroRestore® and Alzstatin® – with a focus on Alzheimer’s disease, where the leading candidate ACD856 is in clinical development phase. Painless – focuses on pain treatment and contains two projects: ACD440 in clinical development phase and TrkA-NAM in research phase.
There are several drug candidates in the various platforms: two candidates in NeuroRestore and two candidates in Alzstatin, as well as two projects that remain in the Painless platform. A diversified portfolio of drug candidates paves the way for other indications, such as cognitive disorders associated with Alzheimer’s, traumatic brain injury, sleep apnea and Parkinson’s disease, as well as for severe pain in conditions such as neuropathy and osteoarthritis.
The NeuroRestore platform is a new generation of symptomatic drugs for the treatment of illnesses with cognitive disorders, such as Alzheimer’s disease. The target mechanism also has other potential indications, including depression and cognitive disorders in Parkinson’s disease, traumatic brain injury and sleep disorders. The leading drug candidate in the project, ACD856, is in clinical development phase.
Innovative small molecule disease-modifying and preventive drugs for Alzheimer’s disease are under development within the Alzstatin platform. They are intended to enable simple administration of the drug and be more cost-effective. The two Alzstatin projects are in the preclinical development phase.
The Painless platform includes two projects: TrkA-NAM and ACD440, which both focus on severe pain.
- The drug candidate ACD440 was in-licensed in January 2020 and affects a specific biological mechanism; the 2021 Nobel Prize in Physiology or Medicine was awarded for the discovery of this The compound is being developed for the treatment of neuropathic pain, a field with great unmet medical need. The project is currently in the clinical development phase.
- The TrkA-NAM project is aimed at treating severe pain caused by disorders such as osteoarthritis, which today lacks sufficiently effective treatment. The project is currently in the research phase.
AlzeCure Pharma’s Pipeline
Research phase: Preclinical discovery and optimization phase. In this phase, potential new drug candidates are synthesized, and their chemical and biological properties are assessed in various in vitro and in vivo test systems. This includes testing of pharmacological effects, pharmacological versus toxicological dose levels, different administration methods and translation to humans.
Pre-clinical development phase: Preparation for clinical studies in humans. The drug candidate undergoes preclinical safety and toxicological tests to determine the tolerability of the drug candidate, adverse reaction profile and maximum dosage level before starting clinical studies. Final formulation work, stability studies and similar work are also carried out. Documentation is compiled and submitted to regulatory authorities and ethics committees for approval before starting clinical studies.
Clinical Phase I: During Phase I, the drug candidate is tested and evaluated from a safety and tolerability perspective, usually in a smaller group of healthy volunteers. The focus from a regulatory perspective is to assess the safety profile, dose levels, how the substance is absorbed, degraded in the body and excreted, as well as evaluating possible interactions with other drugs and food.
Clinical Phase II: In Phase II, the drug candidate is given to patients with the disease to be treated. A primary aim of this clinical phase is to measure efficacy in a relevant patient population, but also to provide additional information on tolerability in the patient population. During this phase, preclinical studies are also being conducted to assess the possibility of giving the drug to pregnant women and children. The intended final formulation for the market is developed.
Clinical Phase III: The aim of Phase III clinical studies is to measure efficacy in a larger patient population with the relevant disease. In Phase III, the efficacy and safety of the drug candidate is confirmed in a patient group that, as far as possible, mimics the population in which the finished drug is to be used, with regard to weight, age, gender, etc. A comparison is made with the current standard treatment of care, or placebo if no standard treatment is available for the disease in question.